
FDA's green list cracks down on illegal imported drug ingredients
The Food and Drug Administration is cracking down on ingredients from potentially from unverified foreign sources and that could be used in GLP-1 drugs that include Wegovy, Ozembic and Mounjaro.

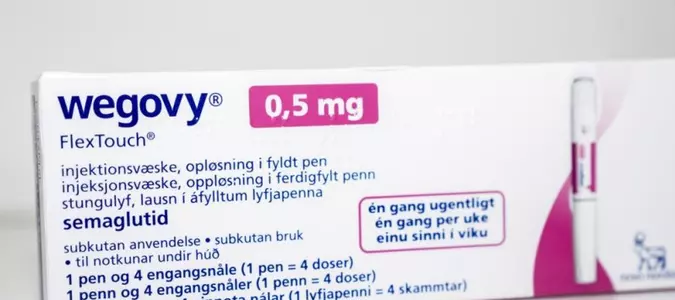
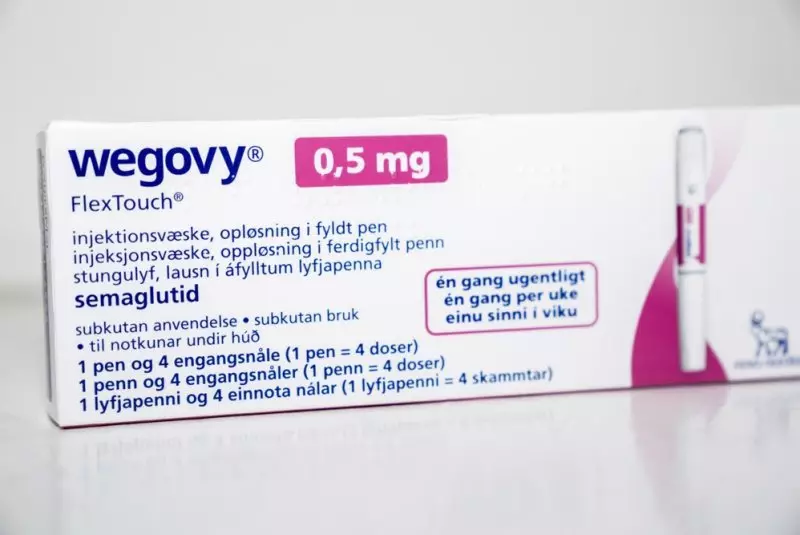
A package of Wegovy by Novo Nordisk sits on a table in Copenhagen, Denmark, in 2023. The FDA is cracking down on ingredients from potentially dangerous active ingredients used in GLP-1 drugs that also include Ozembic, and Mounjaro. File Photo by Ida Marie Odgaard/EPA UPI
Sept. 5 (UPI) -- The Food and Drug Administration is cracking down on ingredients from potentially from unverified foreign sources and that could be used in GLP-1 drugs that include Wegovy, Ozembic and Mounjaro.
The drugs are use for treating Type 2 diabetes and, in certain cases, for weight loss.
The FDA announced Friday it published a "green list" of import alert to stop the unverified foreign sources from coming into the U.S. market. Other GLP-1 drugs, some of which are older medications, include Adlyxin, Byetta, Bydureon, Retatrutide, Rybelsus, Saxenda, Trulicity, Victoza, Zepbound.
"This is part of the agency's decisive steps to safeguard consumers from illegal GLP-1 active ingredients imported from overseas to ensure patient safety and a secure drug supply chain," the FDA said in a news release.
The list of those compliant includes more 37 manufacturers, though their names are redacted. Countries are Belgium, Canada, China, Denmark, Germany, India, Ireland, Italy, Portugal, Sweden, Switzerland and Taiwan.
The FDA has approved certain GLP-1 drugs, including semaglutide and tirzepatide, for medical purposes.
"However, the agency is aware that some patients are turning to compounded versions of these drugs, which are not approved by the FDA," the agency said. "To protect patients who use these compounded drugs, the green list will include GLP-1 APIs from facilities the agency has inspected or evaluated that appear to be in compliance with the FDA's rigorous standards - standards applicable to all APIs manufactured in the U.S.
"APIs from other sources are subject to detention without physical examination."
As of July 31, the FDA said it had 605 reports of adverse events associated with compounded semaglutide and 545 reports of adverse events associated with compounded tirzepatide.
If you receive a product with a licensed pharmacy name on the label that you think might be fraudulent, you are urged to the pharmacy to ask if it is their product.
Previously, the FDA identified serious concerns about compound versions with dosing errors, unapproved salt forms and adverse events, some of which require hospitalization.
"Americans should be confident that the prescription drugs they take are safe," FDA Commissioner Dr. Marty Makary said. "By strengthening oversight of imported APIs and cracking down on illegal drugs entering the U.S., we are taking aggressive action to protect consumers from poor-quality or dangerous GLP-1 drugs."
The FDA said it will continue to work with state regulators, monitor the market and take enforcement actions as necessary.
"Our priority is protecting public health by ensuring all active ingredients used in GLP-1 drugs are obtained from compliant manufacturers," Dr. George Tidmarsh, director of the FDA's Center for Drug Evaluation and Research, said. "Targeting illegal foreign GLP-1 active ingredients at the border is a critical part of this work."
Mounjaro is manufactured by U.S.-headquartered Eli Lilly & Co., and Wegovy is made by Novo Nordisk, based in Denmark. They are both injectables taken weekly.
Both drugs regulate blood sugar and control appetite with the outcome weight loss.
Mounjaro's related drug Zepbound is designed for weight loss only and contains the same active ingredient of tirzepatide.
Wegovy has been approved for Type 1 diabetes and weight loss, with Ozembic containing lower doses of semaglutide.
The FDA approved Wegovy in 2011 and Mounjaro in 2022.
These two companies have mounted legal challenges to block compounders from selling alternative versions of the drugs. Bloomberg reported these versions proliferated when there were shortages of Wegovy and Mounjaro. The drugs are not readily available, though they cost a without insurance.